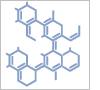
8H-Indeno[1,2-c]thiophen-8-one
Artikel-Nr:
(BOSSBS-2333R-A488)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-2333R-A488
Lokale Artikelnummer::
BOSSBS-2333R-A488
Beschreibung:
Natriuretic peptide receptor C does not exhibit guanylate cyclase activity. There seem to be at least three ANP receptors: two with guanylate cyclase activity (ANPA and ANPB) and one (ANPC) which is probably responsible for the clearance of ANP from the circulation without a role in signal transduction.
VE:
1 * 100 µl
Artikel-Nr:
(BLDPBD01432118-100)
Lieferant:
BLD PHARMATECH GMBH
Hersteller-Artikelnummer::
BD01432118-100
Lokale Artikelnummer::
BLDPBD01432118-100
Beschreibung:
(3,5-Diamino-4-isobutylphenyl)boronic acid 95%
VE:
1 * 100 mg
Lieferant:
Thermo Scientific
Beschreibung:
L-allo-Threonin 99%
Lieferant:
Alfa Aesar
Beschreibung:
4-(Trimethylsilyl)-3-butin-1-ol ≥98%
Artikel-Nr:
(BOSSBS-7109R-CY5.5)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-7109R-CY5.5
Lokale Artikelnummer::
BOSSBS-7109R-CY5.5
Beschreibung:
Component of the vault ribonucleoprotein particle which is at least composed of MVP, PARP4 and one or more vault RNAs (vRNAs). Binds to MVP. Associates with TEP1. Widely expressed; the highest levels are in the kidney; also detected in heart, placenta, lung, liver, skeletal muscle, spleen, leukocytes and pancreas.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-7109R-CY7)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-7109R-CY7
Lokale Artikelnummer::
BOSSBS-7109R-CY7
Beschreibung:
Component of the vault ribonucleoprotein particle which is at least composed of MVP, PARP4 and one or more vault RNAs (vRNAs). Binds to MVP. Associates with TEP1. Widely expressed; the highest levels are in the kidney; also detected in heart, placenta, lung, liver, skeletal muscle, spleen, leukocytes and pancreas.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-12260R-CY7)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-12260R-CY7
Lokale Artikelnummer::
BOSSBS-12260R-CY7
Beschreibung:
The proteasome represents a large protein complex that exists inside all eukaryotes and archaea, and in some bacteria. The main function of proteasomes is to degrade unnecessary or damaged proteins by proteolysis. The most common form of the proteasome, known as the 26S Proteasome, contains one 20S Proteasome core particle structure and two 19S regulatory caps. The 20S Proteasome core is hollow and forms an enclosed cavity, where proteins are degraded, as well as openings at the two ends to allow the target protein to enter. The 20S Proteasome core particle contains many subunits, depending on the organism. All of the subunits fall into one of two types: alpha subunits, which are structural, serve as docking domains for the regulatory particles and exterior gates blocking unregulated access to the interior cavity; or beta subunits, which are predominantly catalytic. The outer two rings in the proteasome consist of seven ?subunits each, and the inner two rings each consist of seven beta subunits.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-12574R-A488)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-12574R-A488
Lokale Artikelnummer::
BOSSBS-12574R-A488
Beschreibung:
p130 represents one of several known substrates for v-Crk encoded p47. p130 Cas (for Crk-associated substrate) exhibits a high level of tyrosine phosphorylation and is tightly associated with v-Crk, suggesting a role in v-Crk-mediated cell signaling. The molecular cloning of p130 Cas has shown it to represent a novel SH3 containing signaling molecule with a cluster of multiple putative SH2-binding motifs for v-Crk. By immunoprecipitation analysis, p130 Cas has been shown to be highly phosphorylated at tyrosine residues subsequent to either v-Src p60 or v-Crk-mediated transformation and to form stable complexes with both of these transforming proteins. p130 Cas behaves as an extremely potent substrate for protein tyrosine kinases and has been reported to relocate from the cytoplasm to cell membrane upon tyrosine phosphorylation. One proposed model is that the SH2 domain of v-Crk functions to activate c-Src kinase, which in turn phosphorylates p130 Cas.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-12574R-A680)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-12574R-A680
Lokale Artikelnummer::
BOSSBS-12574R-A680
Beschreibung:
p130 represents one of several known substrates for v-Crk encoded p47. p130 Cas (for Crk-associated substrate) exhibits a high level of tyrosine phosphorylation and is tightly associated with v-Crk, suggesting a role in v-Crk-mediated cell Signalling. The molecular cloning of p130 Cas has shown it to represent a novel SH3 containing Signalling molecule with a cluster of multiple putative SH2-binding motifs for v-Crk. By immunoprecipitation analysis, p130 Cas has been shown to be highly phosphorylated at tyrosine residues subsequent to either v-Src p60 or v-Crk-mediated transformation and to form stable complexes with both of these transforming proteins. p130 Cas behaves as an extremely potent substrate for protein tyrosine kinases and has been reported to relocate from the cytoplasm to cell membrane upon tyrosine phosphorylation. One proposed model is that the SH2 domain of v-Crk functions to activate c-Src kinase, which in turn phosphorylates p130 Cas.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-12260R-A647)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-12260R-A647
Lokale Artikelnummer::
BOSSBS-12260R-A647
Beschreibung:
The proteasome represents a large protein complex that exists inside all eukaryotes and archaea, and in some bacteria. The main function of proteasomes is to degrade unnecessary or damaged proteins by proteolysis. The most common form of the proteasome, known as the 26S Proteasome, contains one 20S Proteasome core particle structure and two 19S regulatory caps. The 20S Proteasome core is hollow and forms an enclosed cavity, where proteins are degraded, as well as openings at the two ends to allow the target protein to enter. The 20S Proteasome core particle contains many subunits, depending on the organism. All of the subunits fall into one of two types: alpha subunits, which are structural, serve as docking domains for the regulatory particles and exterior gates blocking unregulated access to the interior cavity; or beta subunits, which are predominantly catalytic. The outer two rings in the proteasome consist of seven ?subunits each, and the inner two rings each consist of seven beta subunits.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13120R-CY3)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13120R-CY3
Lokale Artikelnummer::
BOSSBS-13120R-CY3
Beschreibung:
The exosome is a multi-subunit complex composed of several highly conserved proteins, some of which are 3’ to 5’ exoribonucleases. The complex is involved in a variety of cellular processes and is responsible for degrading unstable mRNAs that contain AU-rich (ARE) elements in their untranslated 3’ region. EXOSC10, also known as PMSCL, PMSCL2, p2, p3, p4, RRP6, Rrp6p, PM-Scl, or PM/Scl-100, is an 885 amino acid protein that contains one HRDC domain and one 3’-5’ enonuclease domain. Localized to both the cytoplasm and the nucleus, EXOSC10 is part of the post-splicing exosome complex and is involved in mRNA surveillance, mRNA nuclear export and nonsense-mediated decay of mRNAs containing premature stop codons. against EXOSC10 have been found in patients with scleroderma and/or polymyositis (chronic diseases of the skin and muscle, respectively), suggesting that EXOSC10 may be involved in the pathogenesis of these diseases. Two isoforms of EXOSC10 exist due to alternative splicing events.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-12260R-A680)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-12260R-A680
Lokale Artikelnummer::
BOSSBS-12260R-A680
Beschreibung:
The proteasome represents a large protein complex that exists inside all eukaryotes and archaea, and in some bacteria. The main function of proteasomes is to degrade unnecessary or damaged proteins by proteolysis. The most common form of the proteasome, known as the 26S Proteasome, contains one 20S Proteasome core particle structure and two 19S regulatory caps. The 20S Proteasome core is hollow and forms an enclosed cavity, where proteins are degraded, as well as openings at the two ends to allow the target protein to enter. The 20S Proteasome core particle contains many subunits, depending on the organism. All of the subunits fall into one of two types: alpha subunits, which are structural, serve as docking domains for the regulatory particles and exterior gates blocking unregulated access to the interior cavity; or beta subunits, which are predominantly catalytic. The outer two rings in the proteasome consist of seven subunits each, and the inner two rings each consist of seven beta subunits.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13120R-A647)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13120R-A647
Lokale Artikelnummer::
BOSSBS-13120R-A647
Beschreibung:
The exosome is a multi-subunit complex composed of several highly conserved proteins, some of which are 3’ to 5’ exoribonucleases. The complex is involved in a variety of cellular processes and is responsible for degrading unstable mRNAs that contain AU-rich (ARE) elements in their untranslated 3’ region. EXOSC10, also known as PMSCL, PMSCL2, p2, p3, p4, RRP6, Rrp6p, PM-Scl, or PM/Scl-100, is an 885 amino acid protein that contains one HRDC domain and one 3’-5’ enonuclease domain. Localized to both the cytoplasm and the nucleus, EXOSC10 is part of the post-splicing exosome complex and is involved in mRNA surveillance, mRNA nuclear export and nonsense-mediated decay of mRNAs containing premature stop codons. against EXOSC10 have been found in patients with scleroderma and/or polymyositis (chronic diseases of the skin and muscle, respectively), suggesting that EXOSC10 may be involved in the pathogenesis of these diseases. Two isoforms of EXOSC10 exist due to alternative splicing events.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13120R-CY7)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13120R-CY7
Lokale Artikelnummer::
BOSSBS-13120R-CY7
Beschreibung:
The exosome is a multi-subunit complex composed of several highly conserved proteins, some of which are 3’ to 5’ exoribonucleases. The complex is involved in a variety of cellular processes and is responsible for degrading unstable mRNAs that contain AU-rich (ARE) elements in their untranslated 3’ region. EXOSC10, also known as PMSCL, PMSCL2, p2, p3, p4, RRP6, Rrp6p, PM-Scl, or PM/Scl-100, is an 885 amino acid protein that contains one HRDC domain and one 3’-5’ enonuclease domain. Localized to both the cytoplasm and the nucleus, EXOSC10 is part of the post-splicing exosome complex and is involved in mRNA surveillance, mRNA nuclear export and nonsense-mediated decay of mRNAs containing premature stop codons. against EXOSC10 have been found in patients with scleroderma and/or polymyositis (chronic diseases of the skin and muscle, respectively), suggesting that EXOSC10 may be involved in the pathogenesis of these diseases. Two isoforms of EXOSC10 exist due to alternative splicing events.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-12574R-A350)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-12574R-A350
Lokale Artikelnummer::
BOSSBS-12574R-A350
Beschreibung:
p130 represents one of several known substrates for v-Crk encoded p47. p130 Cas (for Crk-associated substrate) exhibits a high level of tyrosine phosphorylation and is tightly associated with v-Crk, suggesting a role in v-Crk-mediated cell signaling. The molecular cloning of p130 Cas has shown it to represent a novel SH3 containing signaling molecule with a cluster of multiple putative SH2-binding motifs for v-Crk. By immunoprecipitation analysis, p130 Cas has been shown to be highly phosphorylated at tyrosine residues subsequent to either v-Src p60 or v-Crk-mediated transformation and to form stable complexes with both of these transforming proteins. p130 Cas behaves as an extremely potent substrate for protein tyrosine kinases and has been reported to relocate from the cytoplasm to cell membrane upon tyrosine phosphorylation. One proposed model is that the SH2 domain of v-Crk functions to activate c-Src kinase, which in turn phosphorylates p130 Cas.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-11210R-A350)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-11210R-A350
Lokale Artikelnummer::
BOSSBS-11210R-A350
Beschreibung:
Dyneins are multisubunit, high molecular weight ATPases that interact with microtubules to generate force by converting the chemical energy of ATP into the mechanical energy of movement. Cytoplasmic or axonemal Dynein heavy, intermediate, light and light-intermediate chains are all components of minus end-directed motors; the complex transports cellular cargos towards the central region of the cell. Axonemal dynein motors contain one to three non-identical heavy chains and cause a sliding of microtubules in the axonemes of cilia and flagella in a mechanism necessary for cilia to beat and propel the cell. DNAH9 (Dynein, axonemal, heavy chain 9), also known as DYH9, HL20, DNEL1, Dnahc9 or DNAH17L, is a member of the Dynein heavy chain family and comprises one of the heavy chain subunits of axonemal Dynein. DNAH9 consists of an N-terminal stem which is responsible for interacting with other Dynein components and binding cargo, and four P-loops that comprise the motor domain at its C-terminus.
VE:
1 * 100 µl
Preis auf Anfrage
Lager für diesen Artikel ist begrenzt, kann aber in einem Lagerhaus in Ihrer Nähe zur Verfügung. Bitte stellen Sie sicher, dass Sie in sind angemeldet auf dieser Seite, so dass verfügbare Bestand angezeigt werden können. Wenn das
Lager für diesen Artikel ist begrenzt, kann aber in einem Lagerhaus in Ihrer Nähe zur Verfügung. Bitte stellen Sie sicher, dass Sie in sind angemeldet auf dieser Seite, so dass verfügbare Bestand angezeigt werden können. Wenn das
Dieses Produkt kann nur an eine Lieferadresse versandt werden die über die entsprechende Lizenzen verfügt. Für weitere Hilfe bitte kontaktieren Sie Ihr VWR Vertriebszentrum.
-Additional Documentation May be needed to purchase this item. A VWR representative will contact you if needed.
Dieses Produkt wurde von Ihrer Organisation gesperrt. Bitte kontaktieren Sie Ihren Einkauf für weitere Informationen.
Dieses Produkt ist Ersatz für den von Ihnen gewünschten Artikel.
Dieses Produkt ist nicht mehr verfügbar. Bitte kontaktieren Sie den VWR Kundenservice.
|
|||||||||