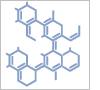
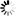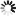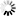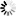2-(Carboxymethyl)-6-fluorobenzoic+acid
Final fill for mAbs
Final fill for mAbs Progressing from the drug substance to the final injectable drug product requires final packaging vials and stoppers that meet a number of quality requirements. To meet these requirements products must be prepared in an ISO Cla...
Final fill for vaccines
Final fill for vaccines Progressing from the drug substance to the final injectable drug product requires final packaging vials and stoppers that meet a number of quality requirements. To meet these requirements products must be prepared in an ISO...
Upstream processing for cell therapy
Upstream processing for cell therapy Compared to production volumes associated with other biologics (e.g. mAbs: 2000L to 10000L) cell therapy production volumes are more commonly between 1L to 10L. Even the quality parameters are different. Instea...
Real-Time PCR (qPCR): An Overview | VWR
Real-Time PCR (qPCR): A Comprehensive Overview A real-time polymerase chain reaction (PCR or qPCR) is a molecular biology laboratory technique based on the polymerase chain reaction (PCR). It monitors the amplification of a specific DNA molecule d...
Chemical_CapabilityTable_v011.indd
Chemical_CapabilityTable_v011.indd For Research Use Only © 2016 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. PFLSPSTORAGECHEM 0716 Chemical Compatibility ...
Final fill for cell therapy
Final fill for cell therapy The cell therapy drug product is configured in different ways depending on the required delivery of the cells/tissues. For example delivery of chondrocytes for cartilage repair is administered by an injection. Expanded ...
Downstream purification for mAbs
Downstream purification for mAbs Major processes including affinity ion exchange HIC and size exclusion require chromatography resins and process buffers. These products help ensure that the quality of the target mAb is maintained through each ste...
Upstream processing for microbial-based recombinant proteins
Upstream processing for microbial-based recombinant proteins Using microbial fermentation for the development and manufacture of recombinant proteins keeps on showing advantages e.g for the expression of proteins that do not require post-translati...
Montreal Manufacturing Facility
Montreal Manufacturing Facility The off-loading manifold at our Montreal QC manufacturing facility which includes 8600 sq. ft. for manufacturing 2200 sq. ft. for quality control and a 22800 sq. ft. warehouse. Core Capabilities Production of labora...
Upstream processing for mAbs
Upstream processing for mAbs Developing and manufacturing mAbs has moved away from a dependency on raw materials of animal origin. Our biochemical components and supplements are animal component-free to meet the needs of these applications. Produc...
Avantor Services Clinical Laboratory Space Optimiz
Success story Avantor Services Lab & production services: business process consulting PROBLEM A large clinical laboratory was challenged by limited space as well as safety and ergonomic risk factors. SOLUTION An Avantor Services consultant was eng...
Safety solutions
Safety solutions Protecting your staff is a top priority and Avantor can help you source necessary products to ensure workplace safety. From head-to-toe personal protection equipment specialized storage and disposal receptacles to critical materia...
Upstream processing for vaccines
Upstream processing for vaccines Optimal production of vaccines is achieved in mammalian expression and insect systems using cell lines that are anchorage-dependent or suspension enabled. For most vaccines optimal production is achieved in mammali...
COL04285-RapidGoldBCA-whitepaper-Final
WHITE PAPER Pierce Rapid Gold BCA Protein Assay A rapid sensitive protein assay for the accurate analysis of protein concentrations rapidly at room temperature (RT) to provide ready-to-read results within 5 minutes without the need for incubation ...
(Microsoft PowerPoint - Pr\344sentation VWR ChromJournal_Chromolith WP Epoxy-Immobilization_062018-v
(Microsoft PowerPoint - Pr\344sentation VWR ChromJournal_Chromolith WP Epoxy-Immobilization_062018-vs2_BP \(005\).PPTX [Read-Only]) Benjamin Peters Tom Kupfer Gisela Jung Peter Knoell Egidijus Machtejevas Petra Lewits Juni 2018 via immobilization ...
product-manual-TotalPure-NGS
Mag-Bind® Total Pure NGS M1378-00 5 mL M1378-01 50 mL M1378-02 500 mL Manual Date: November 2018 Revision Number: v2.1 1 Introduction and Overview.......................................................2 Illustrated Protocol...........................
|
865 - 533 of 533
|
||
|---|---|---|