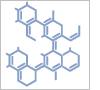
2-Brom-5-fluorisonicotins\\u00E4ure
Artikel-Nr:
(BOSSBS-0315R-FITC)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-0315R-FITC
Lokale Artikelnummer::
BOSSBS-0315R-FITC
Beschreibung:
FGL2 is a secreted protein that is similar to the beta- and gamma-chains of fibrinogen. The carboxyl-terminus of the encoded protein consists of the fibrinogen-related domains (FRED). The encoded protein forms a tetrameric complex which is stabilized by interchain disulfide bonds. It may play a role in physiologic functions at mucosal sites. It is constitutively expressed in cytotoxic T-cells. Lack of expression in other lymphoid- and nonlymphoid-derived cell lines suggested that expression of FGL2 may be restricted to lymphocytes. FGL2 is induced via a mechanism involving IFNG and components of the IFNG signaling pathway, including STAT1 and IRF1.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-0315R-A488)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-0315R-A488
Lokale Artikelnummer::
BOSSBS-0315R-A488
Beschreibung:
FGL2 is a secreted protein that is similar to the beta- and gamma-chains of fibrinogen. The carboxyl-terminus of the encoded protein consists of the fibrinogen-related domains (FRED). The encoded protein forms a tetrameric complex which is stabilized by interchain disulfide bonds. It may play a role in physiologic functions at mucosal sites. It is constitutively expressed in cytotoxic T-cells. Lack of expression in other lymphoid- and nonlymphoid-derived cell lines suggested that expression of FGL2 may be restricted to lymphocytes. FGL2 is induced via a mechanism involving IFNG and components of the IFNG signaling pathway, including STAT1 and IRF1.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-6988R-CY5)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-6988R-CY5
Lokale Artikelnummer::
BOSSBS-6988R-CY5
Beschreibung:
RuBisCO catalyzes two reactions: the carboxylation of D-ribulose 1,5-bisphosphate, the primary event in carbon dioxide fixation, as well as the oxidative fragmentation of the pentose substrate in the photorespiration process. Both reactions occur simultaneously and in competition at the same active site.Sequence similarities: Belongs to the RuBisCO large chain family. Type I subfamily.Post-translational modifications: The disulfide bond which can form between Cys-247 in the large chain dimeric partners within the hexadecamer appears to be associated with oxidative stress and protein turnover (By similarity). The disulfide bonds reported in 1RBO may be the result of oxidation during crystallization.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-1970R-FITC)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-1970R-FITC
Lokale Artikelnummer::
BOSSBS-1970R-FITC
Beschreibung:
Lysosome associated membrane protein (LAMP1), also known as lgp120 or lgpA, is a type 1 integral membrane protein that is transported from trans Golgi networks to endosomes and then lysosomes. Upon cell activation, LAMP1 transfer to the plasma membrane is dependent on a carboxyl terminal tyrosine based motif (YXXI). Perturbation in the spacing between the tyrosine based motif relative to the membrane abolishes lysosome localization of LAMP1. This mutant protein then cycles between the plasma membrane and the endosome. Cell surface LAMP1 and LAMP2 have been shown to promote adhesion of human peripheral blood mononuclear cells (PBMC) to vascular endothelium, therefore they are possibly involved in the adhesion of PBMCs to the site of inflammation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-1970R-CY7)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-1970R-CY7
Lokale Artikelnummer::
BOSSBS-1970R-CY7
Beschreibung:
Lysosome associated membrane protein (LAMP1), also known as lgp120 or lgpA, is a type 1 integral membrane protein that is transported from trans Golgi networks to endosomes and then lysosomes. Upon cell activation, LAMP1 transfer to the plasma membrane is dependent on a carboxyl terminal tyrosine based motif (YXXI). Perturbation in the spacing between the tyrosine based motif relative to the membrane abolishes lysosome localization of LAMP1. This mutant protein then cycles between the plasma membrane and the endosome. Cell surface LAMP1 and LAMP2 have been shown to promote adhesion of human peripheral blood mononuclear cells (PBMC) to vascular endothelium, therefore they are possibly involved in the adhesion of PBMCs to the site of inflammation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-1970R-HRP)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-1970R-HRP
Lokale Artikelnummer::
BOSSBS-1970R-HRP
Beschreibung:
Lysosome associated membrane protein (LAMP1), also known as lgp120 or lgpA, is a type 1 integral membrane protein that is transported from trans Golgi networks to endosomes and then lysosomes. Upon cell activation, LAMP1 transfer to the plasma membrane is dependent on a carboxyl terminal tyrosine based motif (YXXI). Perturbation in the spacing between the tyrosine based motif relative to the membrane abolishes lysosome localization of LAMP1. This mutant protein then cycles between the plasma membrane and the endosome. Cell surface LAMP1 and LAMP2 have been shown to promote adhesion of human peripheral blood mononuclear cells (PBMC) to vascular endothelium, therefore they are possibly involved in the adhesion of PBMCs to the site of inflammation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13224R-A350)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13224R-A350
Lokale Artikelnummer::
BOSSBS-13224R-A350
Beschreibung:
Activation of FUSE, the far upstream element, is required for the proper ex-pression of the mammalian gene c-Myc in undifferentiated cells. The binding of FBP1 (FUSE-binding protein or far upstream element-binding protein) to FUSE is necessary for c-Myc expression, indicating that FBP1 functions as a growth-dependent regulator of c-Myc expression. Isolated from proliferating HL-60 cells, FBP1 (FBP), FBP2 and FBP3 comprise a family of single-stranded DNA-binding proteins that specifically bind to FUSE elements. The FBP transcription factors share a conserved central DNA-binding domain and show significant homology in their carboxyl-terminal activation domains. Expression of FBP1 is detected in undifferentiated cells and is substantially decreased following cellular differentiation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13224R-A488)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13224R-A488
Lokale Artikelnummer::
BOSSBS-13224R-A488
Beschreibung:
Activation of FUSE, the far upstream element, is required for the proper ex-pression of the mammalian gene c-Myc in undifferentiated cells. The binding of FBP1 (FUSE-binding protein or far upstream element-binding protein) to FUSE is necessary for c-Myc expression, indicating that FBP1 functions as a growth-dependent regulator of c-Myc expression. Isolated from proliferating HL-60 cells, FBP1 (FBP), FBP2 and FBP3 comprise a family of single-stranded DNA-binding proteins that specifically bind to FUSE elements. The FBP transcription factors share a conserved central DNA-binding domain and show significant homology in their carboxyl-terminal activation domains. Expression of FBP1 is detected in undifferentiated cells and is substantially decreased following cellular differentiation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13225R)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13225R
Lokale Artikelnummer::
BOSSBS-13225R
Beschreibung:
Activation of FUSE, the far-upstream element, is required for the proper ex-pression of the mammalian gene c-Myc in undifferentiated cells. The binding of FBP (FUSE-binding protein or Far upstream element binding protein) to FUSE is necessary for c-Myc expression, indicating that FBP functions as a growth-dependent regulator of c-Myc expression. Isolated from proliferating HL60 cells, FBP, FBP2, and FBP3 comprise a family of single-stranded DNA-binding proteins that specifically bind to FUSE elements. The FBP transcription factors share a conserved central DNA-binding domain and show significant homology in their carboxyl-terminal activation domains. Expression of FBP is detected in undifferentiated cells and is substantially decreased following cellular differentiation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-1970R-A680)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-1970R-A680
Lokale Artikelnummer::
BOSSBS-1970R-A680
Beschreibung:
Lysosome associated membrane protein (LAMP1), also known as lgp120 or lgpA, is a type 1 integral membrane protein that is transported from trans Golgi networks to endosomes and then lysosomes. Upon cell activation, LAMP1 transfer to the plasma membrane is dependent on a carboxyl terminal tyrosine based motif (YXXI). Perturbation in the spacing between the tyrosine based motif relative to the membrane abolishes lysosome localisation of LAMP1. This mutant protein then cycles between the plasma membrane and the endosome. Cell surface LAMP1 and LAMP2 have been shown to promote adhesion of human peripheral blood mononuclear cells (PBMC) to vascular endothelium, therefore they are possibly involved in the adhesion of PBMCs to the site of inflammation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-1970R-A750)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-1970R-A750
Lokale Artikelnummer::
BOSSBS-1970R-A750
Beschreibung:
Lysosome associated membrane protein (LAMP1), also known as lgp120 or lgpA, is a type 1 integral membrane protein that is transported from trans Golgi networks to endosomes and then lysosomes. Upon cell activation, LAMP1 transfer to the plasma membrane is dependent on a carboxyl terminal tyrosine based motif (YXXI). Perturbation in the spacing between the tyrosine based motif relative to the membrane abolishes lysosome localisation of LAMP1. This mutant protein then cycles between the plasma membrane and the endosome. Cell surface LAMP1 and LAMP2 have been shown to promote adhesion of human peripheral blood mononuclear cells (PBMC) to vascular endothelium, therefore they are possibly involved in the adhesion of PBMCs to the site of inflammation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13224R-CY7)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13224R-CY7
Lokale Artikelnummer::
BOSSBS-13224R-CY7
Beschreibung:
Activation of FUSE, the far upstream element, is required for the proper ex-pression of the mammalian gene c-Myc in undifferentiated cells. The binding of FBP1 (FUSE-binding protein or far upstream element-binding protein) to FUSE is necessary for c-Myc expression, indicating that FBP1 functions as a growth-dependent regulator of c-Myc expression. Isolated from proliferating HL-60 cells, FBP1 (FBP), FBP2 and FBP3 comprise a family of single-stranded DNA-binding proteins that specifically bind to FUSE elements. The FBP transcription factors share a conserved central DNA-binding domain and show significant homology in their carboxyl-terminal activation domains. Expression of FBP1 is detected in undifferentiated cells and is substantially decreased following cellular differentiation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13224R-A555)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13224R-A555
Lokale Artikelnummer::
BOSSBS-13224R-A555
Beschreibung:
Activation of FUSE, the far upstream element, is required for the proper ex-pression of the mammalian gene c-Myc in undifferentiated cells. The binding of FBP1 (FUSE-binding protein or far upstream element-binding protein) to FUSE is necessary for c-Myc expression, indicating that FBP1 functions as a growth-dependent regulator of c-Myc expression. Isolated from proliferating HL-60 cells, FBP1 (FBP), FBP2 and FBP3 comprise a family of single-stranded DNA-binding proteins that specifically bind to FUSE elements. The FBP transcription factors share a conserved central DNA-binding domain and show significant homology in their carboxyl-terminal activation domains. Expression of FBP1 is detected in undifferentiated cells and is substantially decreased following cellular differentiation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13224R-HRP)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13224R-HRP
Lokale Artikelnummer::
BOSSBS-13224R-HRP
Beschreibung:
Activation of FUSE, the far upstream element, is required for the proper ex-pression of the mammalian gene c-Myc in undifferentiated cells. The binding of FBP1 (FUSE-binding protein or far upstream element-binding protein) to FUSE is necessary for c-Myc expression, indicating that FBP1 functions as a growth-dependent regulator of c-Myc expression. Isolated from proliferating HL-60 cells, FBP1 (FBP), FBP2 and FBP3 comprise a family of single-stranded DNA-binding proteins that specifically bind to FUSE elements. The FBP transcription factors share a conserved central DNA-binding domain and show significant homology in their carboxyl-terminal activation domains. Expression of FBP1 is detected in undifferentiated cells and is substantially decreased following cellular differentiation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-13224R-FITC)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-13224R-FITC
Lokale Artikelnummer::
BOSSBS-13224R-FITC
Beschreibung:
Activation of FUSE, the far upstream element, is required for the proper ex-pression of the mammalian gene c-Myc in undifferentiated cells. The binding of FBP1 (FUSE-binding protein or far upstream element-binding protein) to FUSE is necessary for c-Myc expression, indicating that FBP1 functions as a growth-dependent regulator of c-Myc expression. Isolated from proliferating HL-60 cells, FBP1 (FBP), FBP2 and FBP3 comprise a family of single-stranded DNA-binding proteins that specifically bind to FUSE elements. The FBP transcription factors share a conserved central DNA-binding domain and show significant homology in their carboxyl-terminal activation domains. Expression of FBP1 is detected in undifferentiated cells and is substantially decreased following cellular differentiation.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-1970R-A350)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-1970R-A350
Lokale Artikelnummer::
BOSSBS-1970R-A350
Beschreibung:
Lysosome associated membrane protein (LAMP1), also known as lgp120 or lgpA, is a type 1 integral membrane protein that is transported from trans Golgi networks to endosomes and then lysosomes. Upon cell activation, LAMP1 transfer to the plasma membrane is dependent on a carboxyl terminal tyrosine based motif (YXXI). Perturbation in the spacing between the tyrosine based motif relative to the membrane abolishes lysosome localization of LAMP1. This mutant protein then cycles between the plasma membrane and the endosome. Cell surface LAMP1 and LAMP2 have been shown to promote adhesion of human peripheral blood mononuclear cells (PBMC) to vascular endothelium, therefore they are possibly involved in the adhesion of PBMCs to the site of inflammation.
VE:
1 * 100 µl
Preis auf Anfrage
Lager für diesen Artikel ist begrenzt, kann aber in einem Lagerhaus in Ihrer Nähe zur Verfügung. Bitte stellen Sie sicher, dass Sie in sind angemeldet auf dieser Seite, so dass verfügbare Bestand angezeigt werden können. Wenn das
Lager für diesen Artikel ist begrenzt, kann aber in einem Lagerhaus in Ihrer Nähe zur Verfügung. Bitte stellen Sie sicher, dass Sie in sind angemeldet auf dieser Seite, so dass verfügbare Bestand angezeigt werden können. Wenn das
Dieses Produkt kann nur an eine Lieferadresse versandt werden die über die entsprechende Lizenzen verfügt. Für weitere Hilfe bitte kontaktieren Sie Ihr VWR Vertriebszentrum.
-Additional Documentation May be needed to purchase this item. A VWR representative will contact you if needed.
Dieses Produkt wurde von Ihrer Organisation gesperrt. Bitte kontaktieren Sie Ihren Einkauf für weitere Informationen.
Dieses Produkt ist Ersatz für den von Ihnen gewünschten Artikel.
Dieses Produkt ist nicht mehr verfügbar. Bitte kontaktieren Sie den VWR Kundenservice.
|
|||||||||