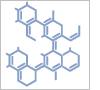
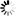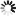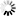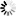4\'-(Dimethylamino)biphenyl-3-carbonsäure
Artikel-Nr:
(BOSSBS-11251R-FITC)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-11251R-FITC
Lokale Artikelnummer::
BOSSBS-11251R-FITC
Beschreibung:
Elucidation of the mechanism by which receptor tyrosine kinases (RTKs) modulate cellular physiology in response to stimuli is critical to the understanding of growth regulation. Miscues in RTK signaling pathways can result in cellular transformation and ultimately in cancer. Two novel EGF receptor substrates designated EGF-receptor pathway substrates 8 and 15, or Eps8 and Eps15, have been described. Eps8 and Eps15 are proteins, respectively that become tyrosine phosphorylated subsequent to EGF stimulation. Overexpression of Eps15 in NIH/3T3 cells causes cellular transformation, implying involvement in the regulation of cell proliferation. Eps15 is capable of binding the amino terminal portion of Crk via a conserved proline-rich domain, characteristic of all Crk binding proteins (5). Overexpression of Eps8 in both fibroblasts and hematopoietic cells results in an increased mitogenic response to EGF. Eps8 has been shown to associate with the EGF receptor despite its lack of a functional SH2 domain. Further characterization suggests the protein has both a PH domain and a SH3 domain, the functional significance of which are not yet known.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-11251R-HRP)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-11251R-HRP
Lokale Artikelnummer::
BOSSBS-11251R-HRP
Beschreibung:
Elucidation of the mechanism by which receptor tyrosine kinases (RTKs) modulate cellular physiology in response to stimuli is critical to the understanding of growth regulation. Miscues in RTK signaling pathways can result in cellular transformation and ultimately in cancer. Two novel EGF receptor substrates designated EGF-receptor pathway substrates 8 and 15, or Eps8 and Eps15, have been described. Eps8 and Eps15 are proteins, respectively that become tyrosine phosphorylated subsequent to EGF stimulation. Overexpression of Eps15 in NIH/3T3 cells causes cellular transformation, implying involvement in the regulation of cell proliferation. Eps15 is capable of binding the amino terminal portion of Crk via a conserved proline-rich domain, characteristic of all Crk binding proteins (5). Overexpression of Eps8 in both fibroblasts and hematopoietic cells results in an increased mitogenic response to EGF. Eps8 has been shown to associate with the EGF receptor despite its lack of a functional SH2 domain. Further characterization suggests the protein has both a PH domain and a SH3 domain, the functional significance of which are not yet known.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-11251R-A750)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-11251R-A750
Lokale Artikelnummer::
BOSSBS-11251R-A750
Beschreibung:
Elucidation of the mechanism by which receptor tyrosine kinases (RTKs) modulate cellular physiology in response to stimuli is critical to the understanding of growth regulation. Miscues in RTK Signalling pathways can result in cellular transformation and ultimately in cancer. Two novel EGF receptor substrates designated EGF-receptor pathway substrates 8 and 15, or Eps8 and Eps15, have been described. Eps8 and Eps15 are proteins, respectively that become tyrosine phosphorylated subsequent to EGF stimulation. Overexpression of Eps15 in NIH/3T3 cells causes cellular transformation, implying involvement in the regulation of cell proliferation. Eps15 is capable of binding the amino terminal portion of Crk via a conserved proline-rich domain, characteristic of all Crk binding proteins. Overexpression of Eps8 in both fibroblasts and hematopoietic cells results in an increased mitogenic response to EGF. Eps8 has been shown to associate with the EGF receptor despite its lack of a functional SH2 domain. Further characterisation suggests the protein has both a PH domain and a SH3 domain, the functional significance of which are not yet known.
VE:
1 * 100 µl
Artikel-Nr:
(SIALA52005-25G)
Lieferant:
Sigma-Aldrich
Hersteller-Artikelnummer::
A52005-25G
Lokale Artikelnummer::
SIALA52005-25G
Beschreibung:
2-Amino-4,6-dimethylpyrimidin, Sigma-Aldrich®
VE:
1 * 25 g
Artikel-Nr:
(BOSSBS-11251R-A350)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-11251R-A350
Lokale Artikelnummer::
BOSSBS-11251R-A350
Beschreibung:
Elucidation of the mechanism by which receptor tyrosine kinases (RTKs) modulate cellular physiology in response to stimuli is critical to the understanding of growth regulation. Miscues in RTK signaling pathways can result in cellular transformation and ultimately in cancer. Two novel EGF receptor substrates designated EGF-receptor pathway substrates 8 and 15, or Eps8 and Eps15, have been described. Eps8 and Eps15 are proteins, respectively that become tyrosine phosphorylated subsequent to EGF stimulation. Overexpression of Eps15 in NIH/3T3 cells causes cellular transformation, implying involvement in the regulation of cell proliferation. Eps15 is capable of binding the amino terminal portion of Crk via a conserved proline-rich domain, characteristic of all Crk binding proteins (5). Overexpression of Eps8 in both fibroblasts and hematopoietic cells results in an increased mitogenic response to EGF. Eps8 has been shown to associate with the EGF receptor despite its lack of a functional SH2 domain. Further characterization suggests the protein has both a PH domain and a SH3 domain, the functional significance of which are not yet known.
VE:
1 * 100 µl
Lieferant:
Thermo Scientific
Beschreibung:
7-Amino-4-(trifluormethyl)cumarin
Artikel-Nr:
(J61105.03)
Lieferant:
Alfa Aesar
Hersteller-Artikelnummer::
J61105.03
Lokale Artikelnummer::
ALFAJ61105.03
Beschreibung:
1-(3-Chlorphenyl)-2-[(2-methyl-2-propanyl)amino]-1-propanonhydrochlorid 99%
VE:
1 * 1 g
Lieferant:
Bernd Kraft
Beschreibung:
Karbolfuchsin (Ziehl-Neelsen)
Artikel-Nr:
(BOSSBS-11251R-CY3)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-11251R-CY3
Lokale Artikelnummer::
BOSSBS-11251R-CY3
Beschreibung:
Elucidation of the mechanism by which receptor tyrosine kinases (RTKs) modulate cellular physiology in response to stimuli is critical to the understanding of growth regulation. Miscues in RTK signaling pathways can result in cellular transformation and ultimately in cancer. Two novel EGF receptor substrates designated EGF-receptor pathway substrates 8 and 15, or Eps8 and Eps15, have been described. Eps8 and Eps15 are proteins, respectively that become tyrosine phosphorylated subsequent to EGF stimulation. Overexpression of Eps15 in NIH/3T3 cells causes cellular transformation, implying involvement in the regulation of cell proliferation. Eps15 is capable of binding the amino terminal portion of Crk via a conserved proline-rich domain, characteristic of all Crk binding proteins (5). Overexpression of Eps8 in both fibroblasts and hematopoietic cells results in an increased mitogenic response to EGF. Eps8 has been shown to associate with the EGF receptor despite its lack of a functional SH2 domain. Further characterization suggests the protein has both a PH domain and a SH3 domain, the functional significance of which are not yet known.
VE:
1 * 100 µl
Artikel-Nr:
(PRSI7197P)
Lieferant:
ProSci Inc.
Hersteller-Artikelnummer::
7197P
Lokale Artikelnummer::
PRSI7197P
Beschreibung:
15 amino acids near the amino terminus of human STAT3.
VE:
1 * 50 µG
Artikel-Nr:
(BOSSBS-11251R-A555)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-11251R-A555
Lokale Artikelnummer::
BOSSBS-11251R-A555
Beschreibung:
Elucidation of the mechanism by which receptor tyrosine kinases (RTKs) modulate cellular physiology in response to stimuli is critical to the understanding of growth regulation. Miscues in RTK signaling pathways can result in cellular transformation and ultimately in cancer. Two novel EGF receptor substrates designated EGF-receptor pathway substrates 8 and 15, or Eps8 and Eps15, have been described. Eps8 and Eps15 are proteins, respectively that become tyrosine phosphorylated subsequent to EGF stimulation. Overexpression of Eps15 in NIH/3T3 cells causes cellular transformation, implying involvement in the regulation of cell proliferation. Eps15 is capable of binding the amino terminal portion of Crk via a conserved proline-rich domain, characteristic of all Crk binding proteins (5). Overexpression of Eps8 in both fibroblasts and hematopoietic cells results in an increased mitogenic response to EGF. Eps8 has been shown to associate with the EGF receptor despite its lack of a functional SH2 domain. Further characterization suggests the protein has both a PH domain and a SH3 domain, the functional significance of which are not yet known.
VE:
1 * 100 µl
Artikel-Nr:
(BOSSBS-11251R-CY7)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-11251R-CY7
Lokale Artikelnummer::
BOSSBS-11251R-CY7
Beschreibung:
Elucidation of the mechanism by which receptor tyrosine kinases (RTKs) modulate cellular physiology in response to stimuli is critical to the understanding of growth regulation. Miscues in RTK signaling pathways can result in cellular transformation and ultimately in cancer. Two novel EGF receptor substrates designated EGF-receptor pathway substrates 8 and 15, or Eps8 and Eps15, have been described. Eps8 and Eps15 are proteins, respectively that become tyrosine phosphorylated subsequent to EGF stimulation. Overexpression of Eps15 in NIH/3T3 cells causes cellular transformation, implying involvement in the regulation of cell proliferation. Eps15 is capable of binding the amino terminal portion of Crk via a conserved proline-rich domain, characteristic of all Crk binding proteins (5). Overexpression of Eps8 in both fibroblasts and hematopoietic cells results in an increased mitogenic response to EGF. Eps8 has been shown to associate with the EGF receptor despite its lack of a functional SH2 domain. Further characterization suggests the protein has both a PH domain and a SH3 domain, the functional significance of which are not yet known.
VE:
1 * 100 µl
Artikel-Nr:
(PRSI7423P)
Lieferant:
ProSci Inc.
Hersteller-Artikelnummer::
7423P
Lokale Artikelnummer::
PRSI7423P
Beschreibung:
15 amino acids near the amino terminus of KHDC1.
VE:
1 * 50 µG
Lieferant:
MP Biomedicals
Beschreibung:
Soluble in water and ethanol.
Artikel-Nr:
(BOSSBS-9406R)
Lieferant:
Bioss
Hersteller-Artikelnummer::
BS-9406R
Lokale Artikelnummer::
BOSSBS-9406R
Beschreibung:
Outer dense fibers are filamentous structures located on the outside of the axoneme in the midpiece and principal piece of the mammalian sperm tail. May help to maintain the passive elastic structures and elastic recoil of the sperm tail.Constituting the main cytoskeletal structure of spermatid flagella, outer dense fibers (ODFs) add elastic recoil, stiffness and protection against shear forces during sperm movement. Human ODFs consist of approximately 10 major and at least 15 minor proteins. The major proteins of the ODF include Odf1, Odf2, and Odf3, which compose a family of proteins that are preferentially expressed during mammalian spermiogenesis. Odf3 (outer dense fiber protein 3), also known as sperm tail protein SHIPPO 1 and TISP50 (transcript induced in spermiogenesis protein 50), is a 254 amino acid protein that is expressed during the latter part of spermatogenesis in flagella of elongated spermatids and mature sperm. Odf proteins are directed to their exact subcellular location by Spags, which are characterized as chaperone-like Odf-binding molecules. There are two isoforms of Odf3 that are produced as a result of alternative splicing events.
VE:
1 * 100 µl
Lieferant:
FLUOROCHEM
Beschreibung:
7-Amino-4-(trifluormethyl)cumarin
Preis auf Anfrage
Lager für diesen Artikel ist begrenzt, kann aber in einem Lagerhaus in Ihrer Nähe zur Verfügung. Bitte stellen Sie sicher, dass Sie in sind angemeldet auf dieser Seite, so dass verfügbare Bestand angezeigt werden können. Wenn das
Lager für diesen Artikel ist begrenzt, kann aber in einem Lagerhaus in Ihrer Nähe zur Verfügung. Bitte stellen Sie sicher, dass Sie in sind angemeldet auf dieser Seite, so dass verfügbare Bestand angezeigt werden können. Wenn das
Dieses Produkt kann nur an eine Lieferadresse versandt werden die über die entsprechende Lizenzen verfügt. Für weitere Hilfe bitte kontaktieren Sie Ihr VWR Vertriebszentrum.
-Additional Documentation May be needed to purchase this item. A VWR representative will contact you if needed.
Dieses Produkt wurde von Ihrer Organisation gesperrt. Bitte kontaktieren Sie Ihren Einkauf für weitere Informationen.
Dieses Produkt ist Ersatz für den von Ihnen gewünschten Artikel.
Dieses Produkt ist nicht mehr verfügbar. Bitte kontaktieren Sie den VWR Kundenservice.
|
|||||||||